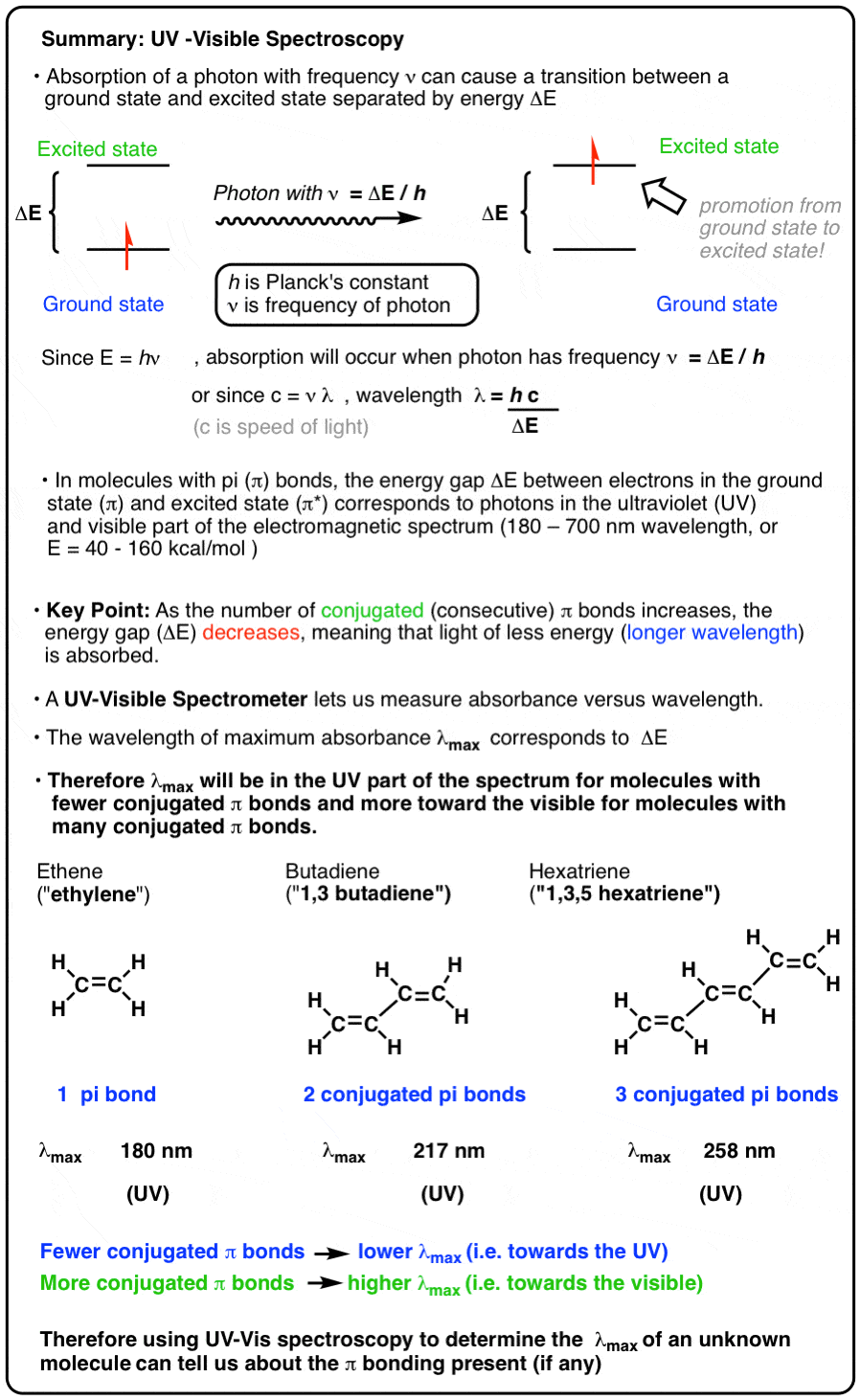
The ultraviolet region is defined as 180 to 400 nm visible between 400 and 800 nm and the near-infrared is from 800 to 3200 nm. ΔE h ν En - E0.
What Is Uv Vis Spectroscopy And How Does It Apply To Conjugation
Near-infrared light is generally poorly absorbed because its photon energy is insufficient to induce electronic transitions and its frequency is.
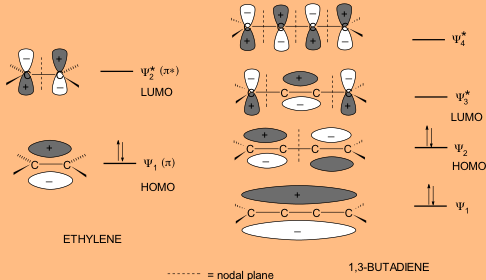
. UV-VisibleNIR spectroscopy can be divided into ultraviolet visible and near-infrared regions of the spectrum. Interaction of EMR with matter 1Electronic Energy Levels. When the molecules absorb UV-visible light from EMR one of the outermost bond lone pair electron is promoted to higher energy state such as E1 E2En etc is called as electronic transition and the difference is as.
At room temperature the molecules are in the lowest energy levels E0.
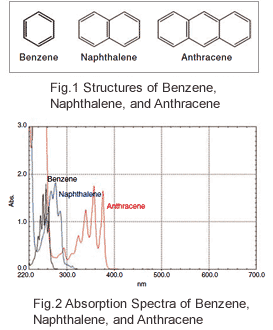
The Relationship Between Uv Vis Absorption And Structure Of Organic Compounds Shimadzu Shimadzu Corporation

Solved Absorption Of Uv Visible Energy By Molecule Results In Vbrational Transilions B Electronic Transitions Rotational Transitions D Nuclear Transitions E None Of The Above Which Of The Following Compounds Absorbs The Longest Wavelength
0 Comments